
Chemotherapy-induced cardiotoxicity (CIC) remains a major clinical challenge in the treatment of acute myeloid leukemia (AML). While anthracyclines such as daunorubicin (DNR) are widely used as first-line agents, their cardiotoxic effects—especially in pediatric and young adult patients—can compromise long-term cardiac function and survival.
A recent study led by Prof. Hao Zhang and Dr. Yiwei Liu, published in European Heart Journal (IF=37.6), uncovers how IL-1α released from tumor cells disrupts cardiac metabolism, contributing to heart dysfunction during chemotherapy.
This research highlights the intercellular crosstalk between tumor and cardiac cells and identifies IL-1α as a key mediator. Crucially, the study demonstrates that neutralizing IL-1α alleviates cardiac injury without compromising anti-leukemic efficacy.
*Service Provider: To investigate cell-specific metabolic changes in patient tissue, the team applied Single-Cell Sequencing Services | 10x-Based CRO – Omics Empower —with technical support from Omics Empower.
Endomyocardial biopsy samples were collected from a representative AML patient who developed heart failure shortly after receiving DNR-based chemotherapy. As a control, tissue was obtained from a brain-dead donor with normal cardiac function.
Single-nucleus RNA sequencing (snRNA-seq) was performed to analyze transcriptional profiles. Eight major cardiac cell types were identified. Differential expression analysis and pseudotime trajectory reconstruction showed that a subset of cardiomyocytes in the AML patient exhibited metabolic dysregulation, characterized by:
Downregulation of genes associated with fatty acid metabolism
Upregulation of genes involved in glucose metabolism
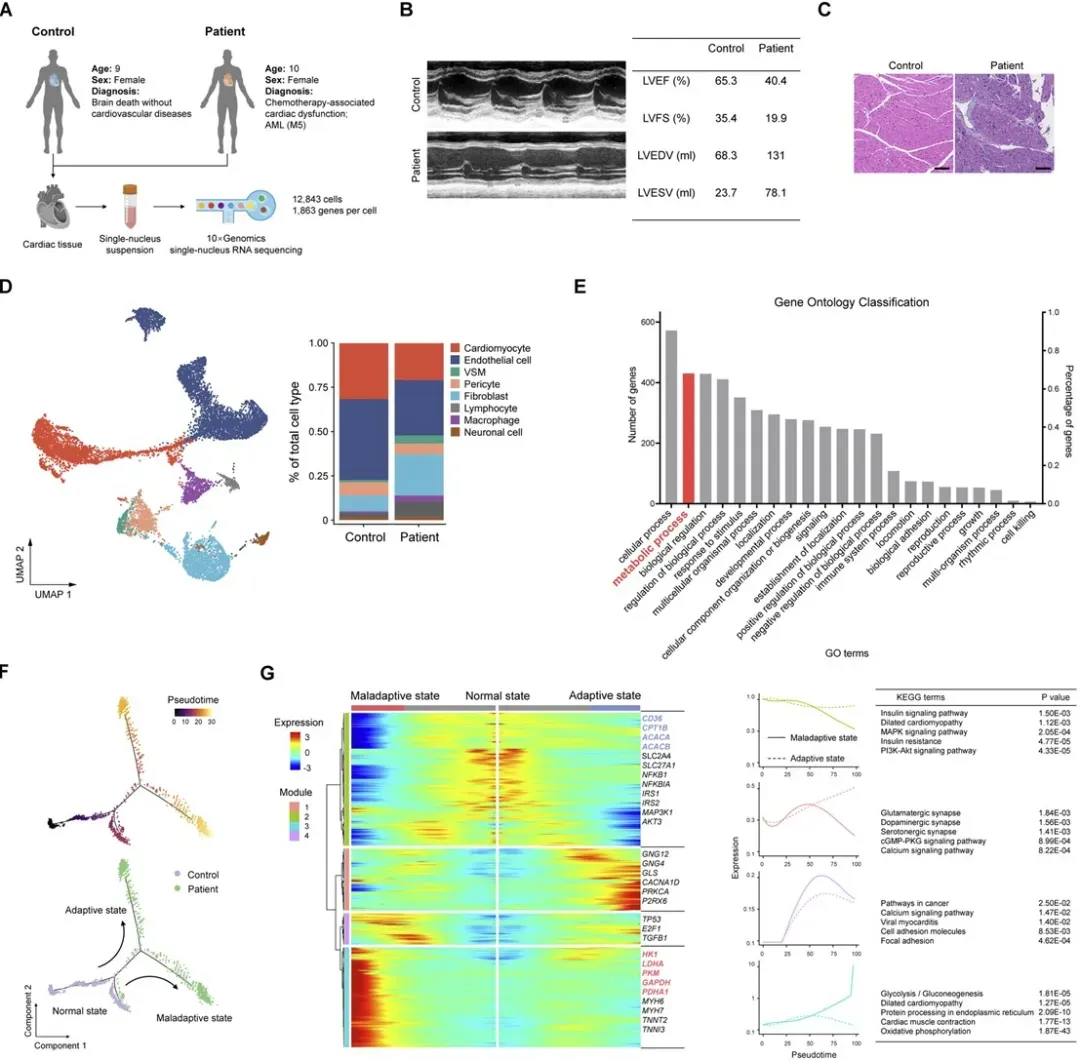
Figure 1. Cardiac metabolic disruption in an AML patient following daunorubicin (DNR) treatment
To model chemotherapy-induced cardiac dysfunction, the researchers established an AML mouse model. Following DNR administration:
Mice exhibited cardiac injury and decreased cardiac function
Compared to untreated AML mice, fatty acid uptake was significantly reduced, while glucose uptake was increased
These mice also displayed signs of cardiac energy deficiency, including:
Reduced ATP levels
Mitochondrial swelling and lower cristae density
Impaired mitochondrial respiration
Importantly, these phenotypes were not observed in tumor-free (TF) mice treated with the same dose of DNR, highlighting the role of leukemia in cardiac metabolic disturbances.
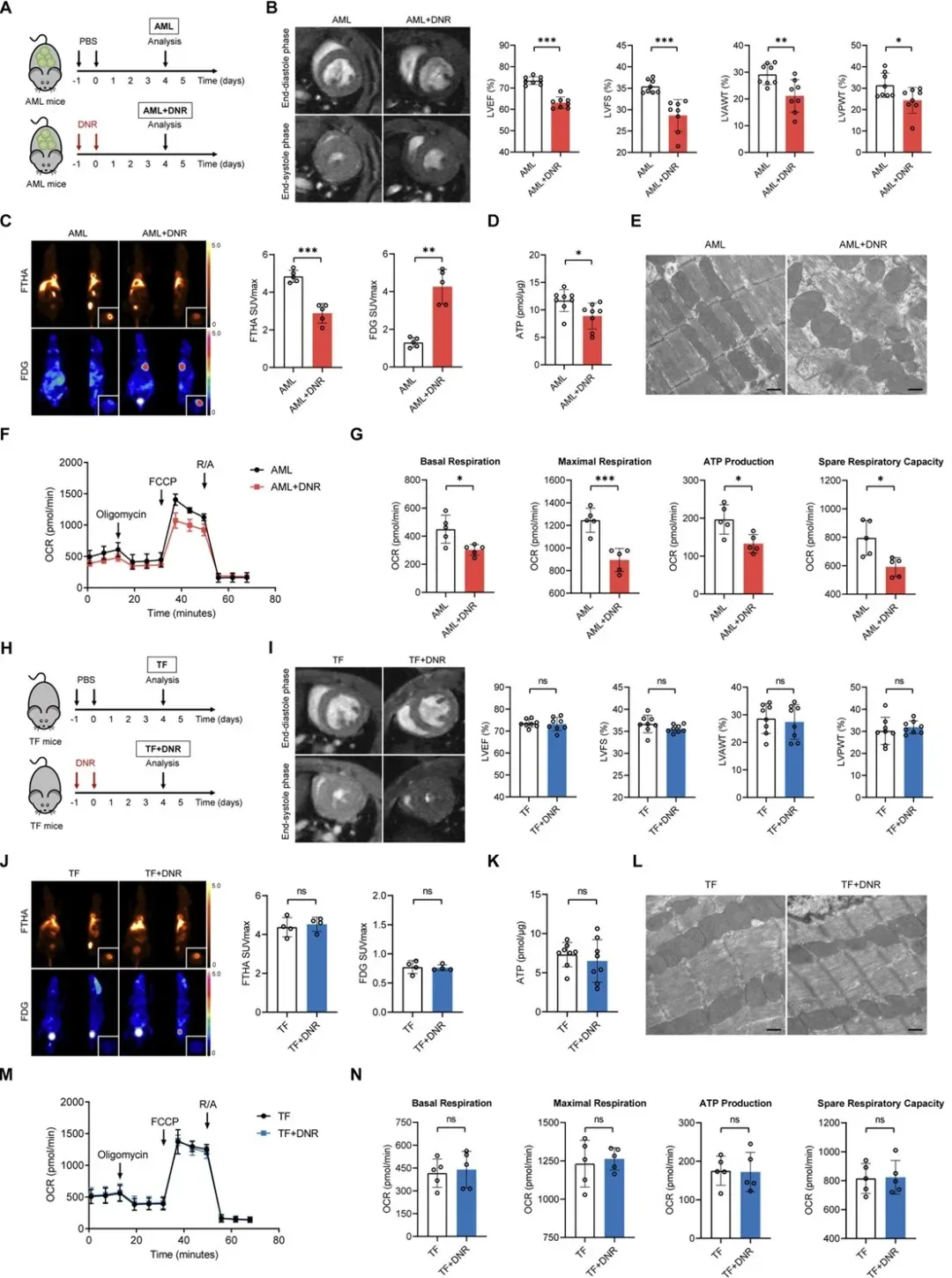
Figure 2. Cardiac metabolic remodeling and functional impairment in AML mice after DNR treatment
To directly investigate cardiac substrate preference during chemotherapy, stable isotope-labeled metabolic flux analysis was performed in AML and AML+DNR mice. The data revealed:
A significant reduction in fatty acid oxidation
Increased glucose utilization as a compensatory response
However, this shift did not fully restore ATP production
Additional analysis showed elevated flux through the pentose phosphate pathway (PPP) and enhanced glucose input into the TCA cycle in DNR-treated AML hearts.

Figure 3. Isotope tracing reveals disrupted fatty acid metabolism in DNR-treated AML mice
Bulk RNA-seq suggested that cytokine signaling may contribute to the observed metabolic disorder in AML+DNR mouse hearts. Subsequent proteomics and immunofluorescence analyses identified interleukin-1 alpha (IL-1α) as a key factor:
IL-1α protein levels were significantly elevated in AML+DNR mice (vs. controls)
Similar increases were observed in cardiac tissue from AML patients
In a patient cohort, plasma IL-1α levels negatively correlated with left ventricular ejection fraction (LVEF)
These findings suggest that tumor-derived IL-1α is involved in chemotherapy-associated cardiac dysfunction, consistent across both animal models and human samples.
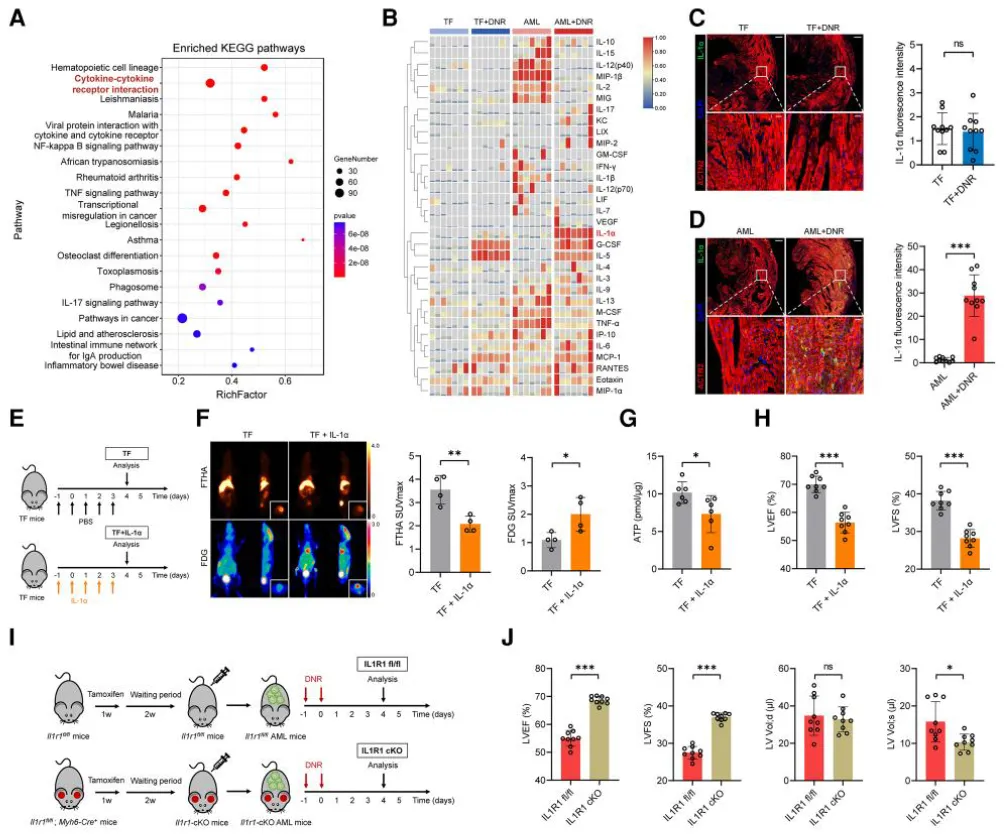
Figure 4. IL-1α mediates metabolic dysfunction in the heart
To identify the source of IL-1α, the authors treated THP-1 leukemia cells with DNR in vitro. Flow cytometry, H&E staining, and immunofluorescence confirmed IL-1α production and release from AML cells post-chemotherapy.
In vivo, the authors used Il1a global knockout mice engrafted with MLL-AF9 AML cells:
IL-1α protein expression in the heart remained unchanged in knockout mice
Cardiac dysfunction persisted after DNR, suggesting that AML cells—not host cells—were the dominant source of IL-1α
Further experiments using shIL-1α-transduced AML cells showed:
Improved cardiac function
Increased myocardial ATP levels
Reduced IL-1α staining in cardiac tissue
These results directly link chemotherapy-induced IL-1α secretion from AML cells to cardiac injury.
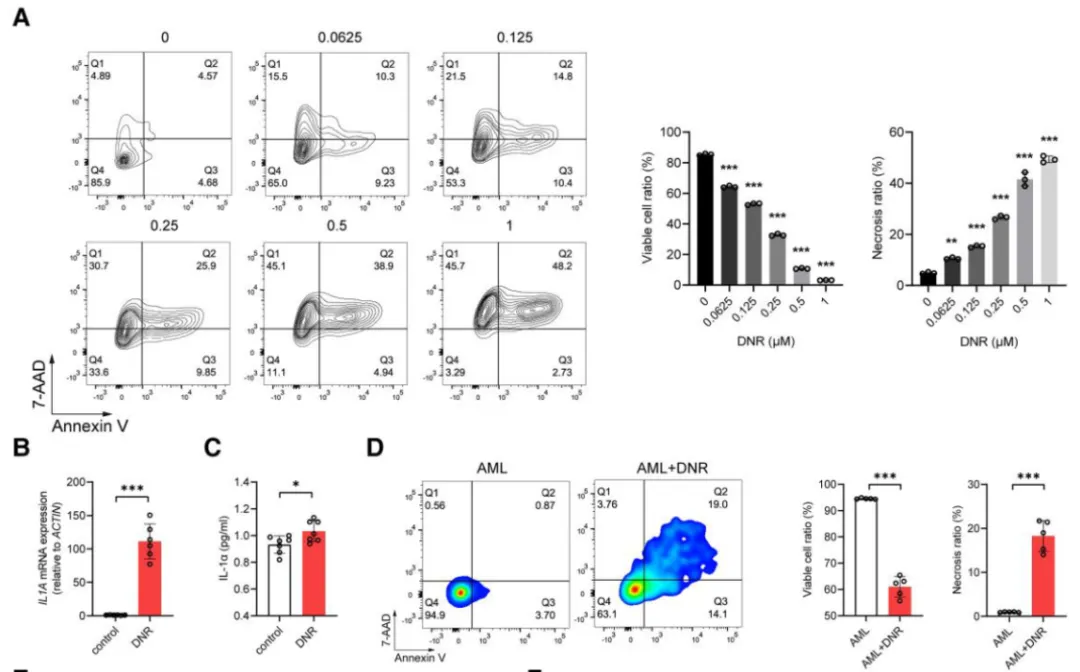
Figure 5. AML cells release IL-1α in response to DNR treatment
Using Ingenuity Pathway Analysis (IPA) and PPI network analysis of heart transcriptomes, the authors proposed that IL-1α activates NF-κB, which in turn suppresses PGC-1α, a key transcriptional regulator of fatty acid metabolism and mitochondrial function.
Western blot and RT-PCR confirmed a negative relationship between NF-κB p65 and PGC-1α expression
In cardiomyocytes exposed to IL-1α, the NF-κB/PGC-1α axis was activated
Overexpression of PGC-1α or inhibition of NF-κB signaling restored mitochondrial function and reversed metabolic defects
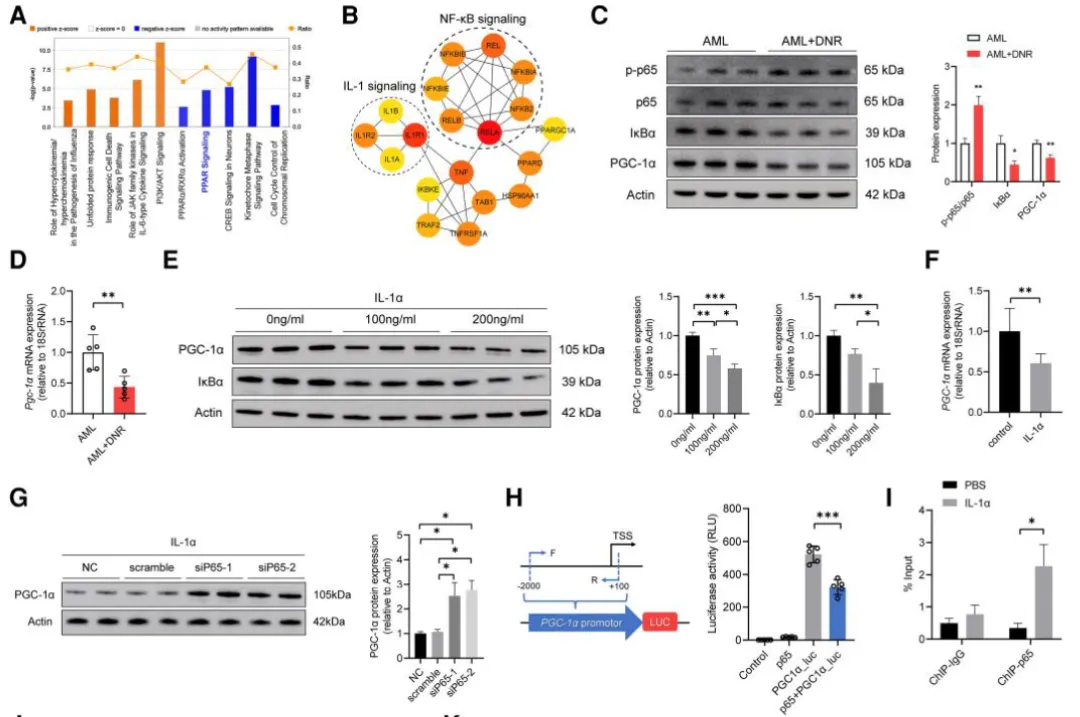
Figure 6. IL-1α–NF-κB–PGC-1α axis regulates cardiac mitochondrial metabolism
To test the therapeutic potential of IL-1α blockade, AML+DNR mice were treated with IL-1α-neutralizing antibodies or IgG controls:
Antibody treatment significantly improved cardiac function
Myocardial ATP levels and mitochondrial function were restored
Expression of PGC-1α and fatty acid metabolism genes increased
Leukemia suppression remained unaffected, confirming that IL-1α blockade did not compromise DNR’s anti-tumor efficacy
These results indicate that targeting IL-1α may be an effective strategy to mitigate chemotherapy-induced cardiotoxicity in AML.
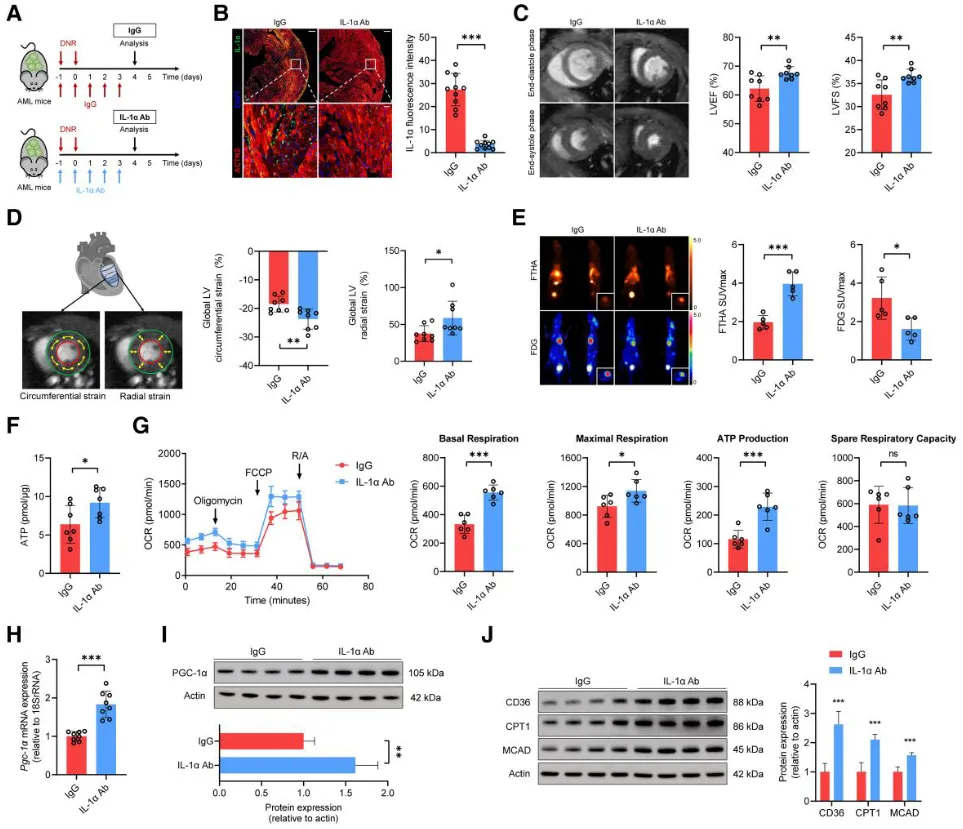
Figure 7. Anti–IL-1α treatment restores cardiac function without affecting chemotherapy efficacy
This study concludes that during daunorubicin (DNR)-based chemotherapy in AML patients, necrosis of tumor cells leads to the release of IL-1α, a pro-inflammatory cytokine. IL-1α activates NF-κB signaling in the heart, which in turn suppresses the expression of PGC-1α—a key regulator of cardiac energy metabolism. This suppression disrupts fatty acid oxidation and reduces energy production, ultimately impairing cardiac function.
Administration of an IL-1α–neutralizing antibody in AML mouse models successfully reversed the chemotherapy-induced metabolic disturbances and restored cardiac function, without compromising the anti-leukemic efficacy of DNR.
These findings suggest that IL-1α blockade may represent a promising therapeutic strategy to reduce cardiotoxicity and improve long-term outcomes for AML patients undergoing chemotherapy.
In this research, single-nucleus RNA sequencing (snRNA-seq) was critical for capturing the transcriptional landscape of human and mouse cardiomyocytes following DNR-based chemotherapy. Omics Empower supported this phase by providing reliable snRNA-seq services, enabling high-resolution mapping of cardiac cell populations and metabolic gene signatures.
Our single-nucleus and single-cell sequencing services are optimized for fragile clinical samples such as heart biopsies, and our bioinformatics team ensures robust downstream analysis tailored to each study's hypothesis.
We have supported over 400 peer-reviewed publications across journals including Nature, Cell, Cancer Cell, and European Heart Journal. Contact us to learn how our single cell sequencing and multi-omics services can accelerate your research.
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong