On March 19, 2025, Professor Tingbo Liang’s team at Zhejiang University published a research article titled “Oncolytic virus VG161 in refractory hepatocellular carcinoma” in Nature. The study found that VG161, an oncolytic virus, demonstrated a favorable safety profile and significant antitumor activity in patients with refractory hepatocellular carcinoma (HCC), suggesting its potential as an effective third-line treatment. The research also offers new directions for the development of oncolytic virus immunotherapy.
*Omics Empower provided bulk RNA-seq, single-cell RNA-seq, single-cell immune repertoire sequencing, and spatial transcriptomics services for this project.If you're looking for similar support in your own research, our expert would be happy to help.
Journal: Nature
Impact Factor: 50.5
Samples:
Multicenter Phase I Clinical Trial: 44 patients (11 dose-escalation; 33 dose-expansion)
Single-cell RNA-seq: 1 surgical sample; biopsy samples from 4 patients (pre- and post-treatment)
Spatial transcriptomics: 1 surgical sample
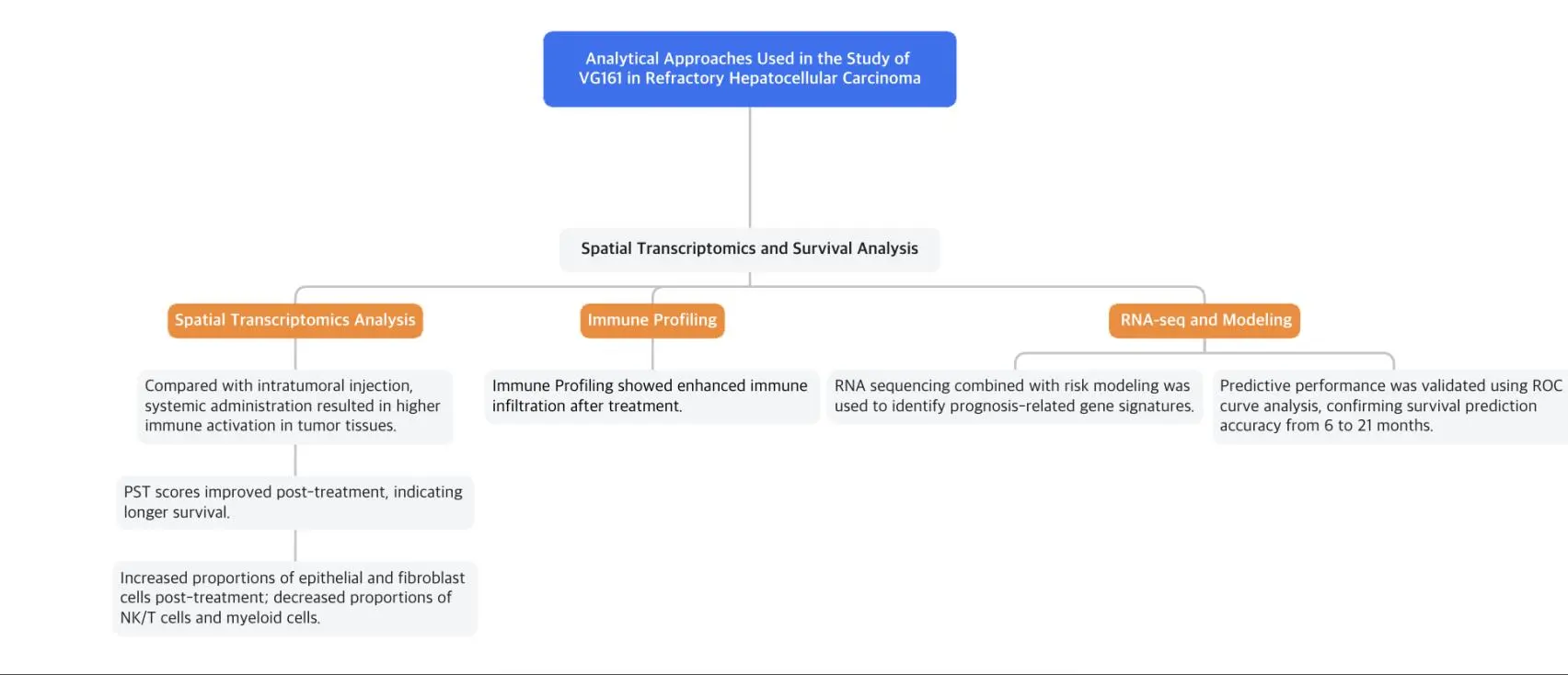
Technical Workflow of the VG161 Study:
Hepatocellular carcinoma is a life-threatening malignancy with limited treatment options after failure of second-line therapies. Oncolytic viruses selectively replicate within and lyse tumor cells, releasing neoantigens that trigger systemic antitumor immunity, offering a potential new therapeutic strategy.
This study reports results from a multicenter Phase I clinical trial evaluating the safety and efficacy of VG161 in advanced HCC patients. VG161 showed good tolerability with no dose-limiting toxicities. Additionally, patients previously sensitive to immune checkpoint inhibitors (CPIs) responded better to VG161. The researchers also developed a predictive model based on differentially expressed genes, which successfully identified patients likely to benefit from VG161 and associated with improved overall survival.
Between April 8, 2021, and November 21, 2023, 44 patients were enrolled across three participating centers. The cohort was divided into a dose-escalation group (11 patients) and a dose-expansion group (33 patients). All participants were included in the safety analysis, while only those who completed at least one tumor assessment were included in the efficacy analysis. The median follow-up as of January 12, 2024, was 7.0 months.
Following intratumoral injection of VG161 in dose-escalation patients, the virus was detectable in blood within 24 hours but declined to undetectable levels afterward. No virus was detected in urine, feces, or saliva. The authors examined whether antiviral medications for hepatitis B affected VG161 efficacy. Among 11 patients (8 HCC and 3 intrahepatic cholangiocarcinoma), 6 took antivirals and 5 did not; no significant difference was found in blood viral DNA levels between the two groups.
In a xenograft mouse model, entecavir did not impair VG161's replication or tumor-killing ability in Hep3B cells.
Significant changes were observed in lymphocyte subpopulations, plasma cytokines, and T cell markers in patients from the dose-escalation group. CD3+, CD4+, and CD8+ cell counts initially dropped 24 hours post-injection but returned to or exceeded baseline levels by Days 15 and 28. TNF and IFN-γ levels rose at 24 hours post-treatment and later returned to baseline. Immune-related markers (e.g., PD-L1, PD-1) increased shortly after dosing and normalized by Days 15 and 28.
VG161 demonstrated a favorable safety profile throughout the trial with no dose-limiting toxicities observed, and the maximum tolerated dose was not reached during dose escalation. The most common treatment-related adverse events (TRAEs) included:
Fever (86.4%)
Hypoalbuminemia and anemia (65.9% each)
Lymphocyte count decrease (84.1%)
Thrombocytopenia and neutropenia (52.3% each)
Leukopenia (70.5%)
Grade 3 lymphopenia was the most frequent severe TRAE. One patient experienced a serious TRAE (facial paralysis), which resolved after proper medical care. No Grade 4 TRAEs or treatment-related deaths were reported.
Due to the limited number of ICC patients (n=4), the efficacy analysis focused on 37 HCC patients. According to modified RECIST (mRECIST) criteria:
7 achieved partial response (PR)
17 had stable disease (SD)
Objective Response Rate (ORR): 18.92%
Disease Control Rate (DCR): 64.86%
After excluding 2 patients with only first-line failure and 1 COVID-19-related death, among the remaining 34 patients:
6 achieved PR
16 had SD
ORR: 17.65%
DCR: 64.71%

Figure 1: Treatment Response Based on mRECIST Criteria
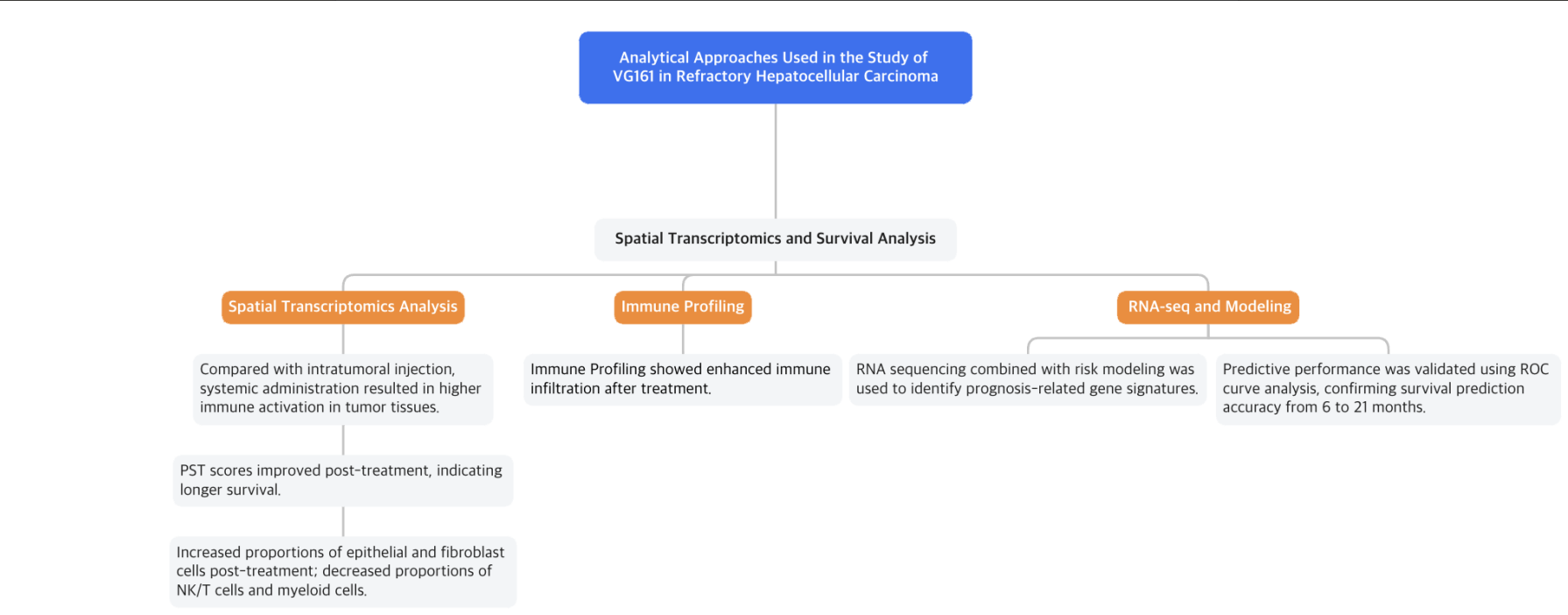
Patients with HCC treated with VG161 showed significant responses, mainly characterized by tumor necrosis (cell death). For example, CT scans of one patient after one treatment cycle revealed that the targeted lesion no longer showed clear enhancement (Fig. 2a). A biopsy confirmed extensive cell death within the lesion, which initially expanded but then showed substantial necrosis. Both injected and non-injected lesions initially increased in size, but as treatment progressed, they developed clear signs of central necrosis, eventually becoming nearly completely necrotic (Fig. 2b).
The researchers performed single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics on a surgical sample from one patient to understand why non-injected lesions responded better to VG161 than injected lesions (Fig. 2e). Combining these analyses, they discovered that injected lesions had more epithelial cells, whereas non-injected lesions had higher levels of NK/T cells and fibroblasts (Fig. 2g, h). Spatial analysis using stLearn software revealed stronger cell-to-cell interactions—especially among NK/T cells—in non-injected lesions, indicating a higher level of immune activation compared to the injected sites (Fig. 2i, j).
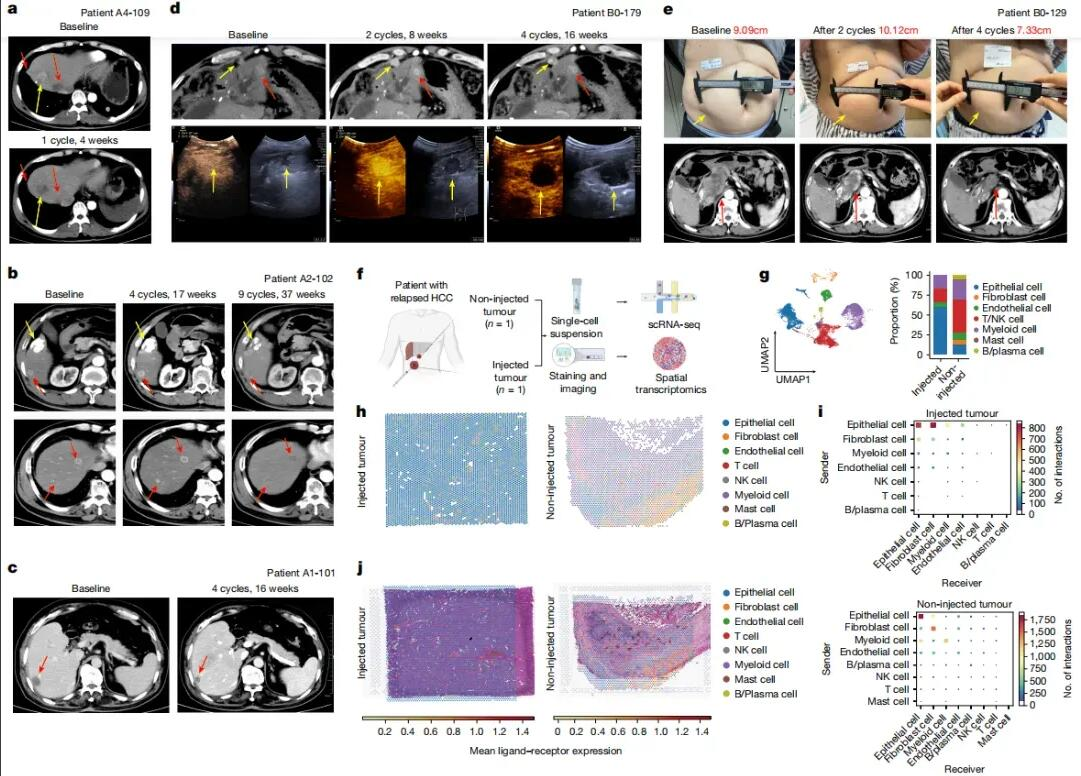
Figure 2: Representative Tumor Response Data from a Responder Patient
Survival analysis was performed on 37 HCC patients with evaluable tumors. The median progression-free survival (PFS) was 2.9 months, and median overall survival (OS) was 12.4 months (Fig. 3a, b). After excluding two patients who had only failed first-line therapy and one patient who died from COVID-19, the median PFS and OS were 2.8 months and 9.40 months, respectively (Fig. 3c, d). Six-month and twelve-month survival rates were 80.2% and 47.8%, respectively.
Patients who had received checkpoint inhibitor (CPI) therapy for three months or less before enrollment (PreCPI ≤3m, n=22) showed significantly improved survival compared to those with longer prior CPI treatment (>3m). In this subgroup, median PFS was 3.6 months versus 1.8 months (Fig. 3e), and median OS significantly improved to 17.30 months versus 7.40 months (Fig. 3f).
Patients who received systemic treatment after VG161 (PST) also had improved survival outcomes (Fig. 3h). Additionally, the use of oral antiviral drugs for hepatitis B virus (HBV) infection did not significantly affect median PFS or OS (Fig. 3i, j). Multivariate analysis identified PreCPI ≤3m and PST as significant independent predictors of overall survival (P = 0.02).
Figure 3: Kaplan-Meier Curves from Baseline
Single-cell immune repertoire sequencing was performed on biopsy samples from four patients to analyze how VG161 treatment affected the tumor microenvironment (TME) of hepatocellular carcinoma (HCC) (Fig. 4a). The analysis identified several cell clusters: four epithelial and fibroblast clusters, one endothelial cell cluster, nine NK/T cell clusters, twelve myeloid cell clusters, one mast cell cluster, and two B/plasma cell clusters (Fig. 4b, c). After treatment, there was an increase in epithelial and fibroblast cell proportions, while NK/T cells and myeloid cells decreased (Fig. 4d–f).
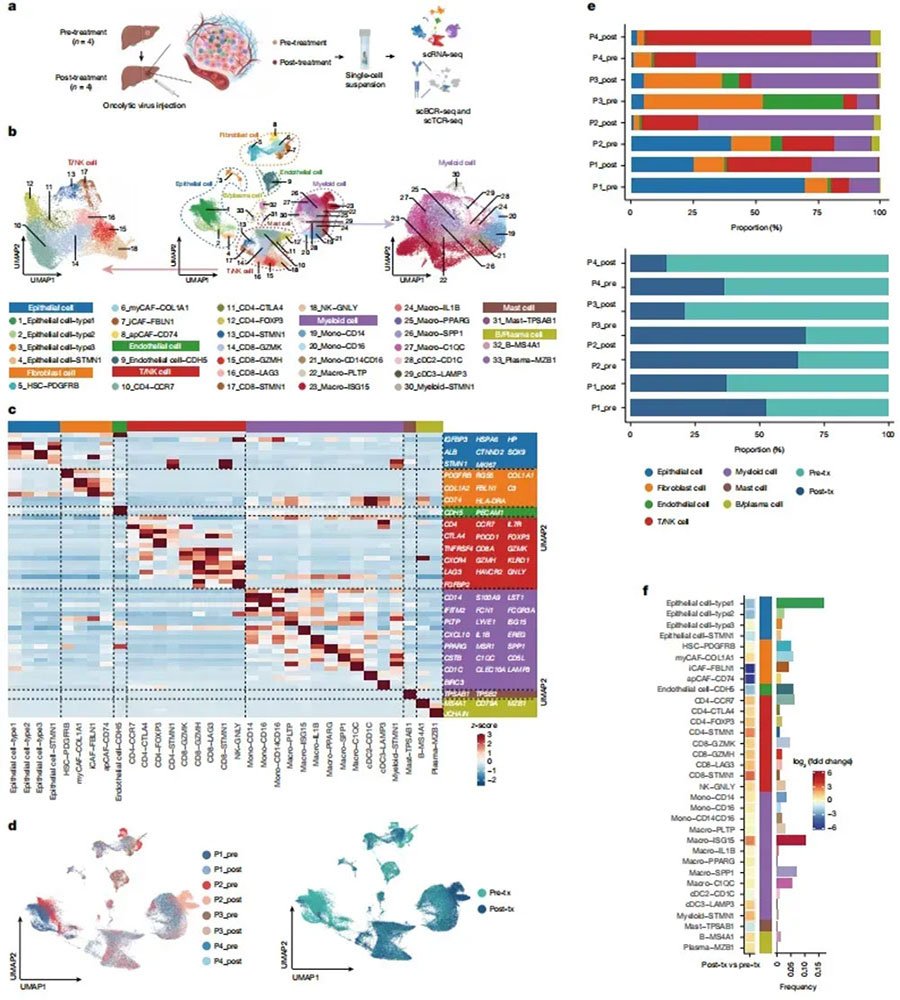
Figure 4: Changes in the immune landscape of the TME after VG161 treatment.
T-cell receptor (TCR) analysis showed significant clonal expansion after treatment, particularly within the CD4-CCR7 and CD8-GZMH T-cell subsets, indicating a strong T-cell immune response (Fig. 5a, b). In contrast, single-cell B-cell receptor sequencing (scBCR-seq) revealed only minor differences in clonal expansion between pre- and post-treatment samples (Fig. 5a, b). Increased expression of immune checkpoint genes after treatment (Fig. 5c) suggested enhanced sensitivity to checkpoint inhibitors (CPIs). Multiplex immunofluorescence imaging confirmed greater infiltration of CD3 and CD8 T cells following VG161 administration, highlighting immune-mediated changes within the TME (Fig. 5e).
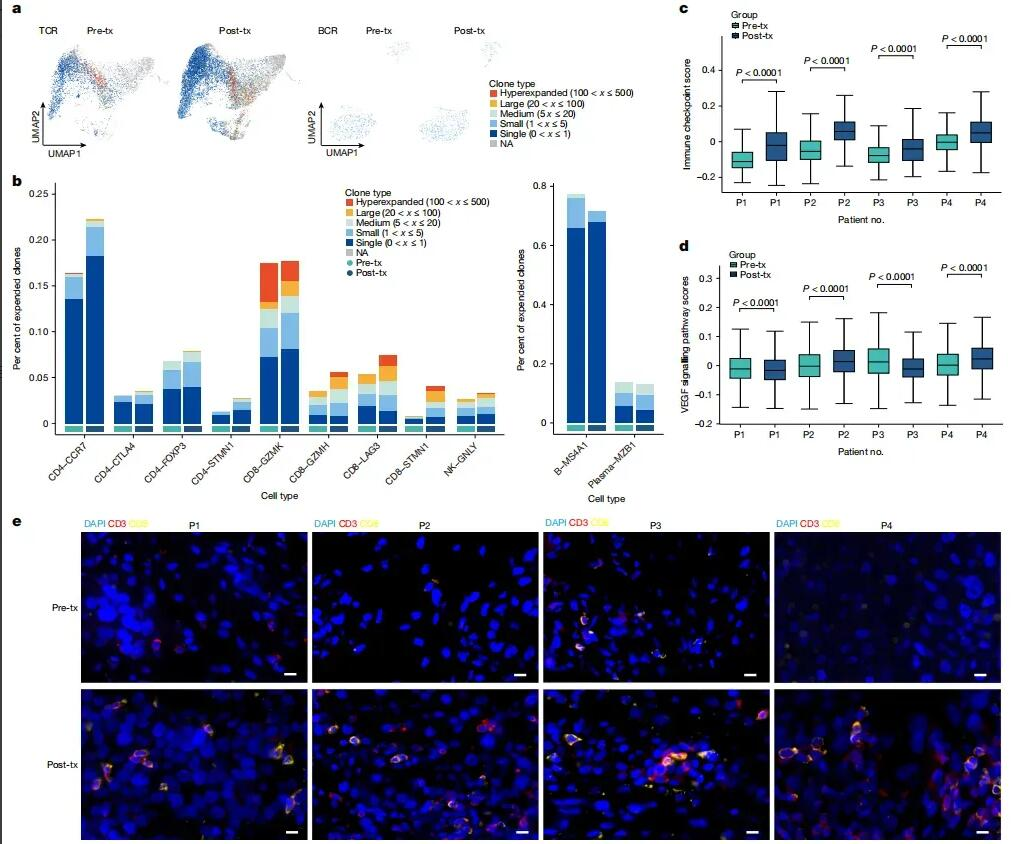
Figure 5: VG161 treatment modulates the TME and enhances T-cell infiltration into tumors.
Baseline tumor samples from 27 patients were analyzed. After excluding 4 patients with intrahepatic cholangiocarcinoma (ICC), 1 patient who had only first-line treatment failure, and 1 patient who died from COVID-19, RNA-seq analysis was conducted on the remaining 21 HCC samples (Fig. 6a). This analysis identified 49 upregulated and 745 downregulated genes, classified according to patient survival based on median overall survival (OS) (Fig. 6b). KEGG and GO analyses indicated enrichment of these genes across several functional categories (Fig. 6d). Univariate Cox regression analysis further identified 299 candidate prognostic genes among these differentially expressed genes (Fig. 6e).
A predictive model for treatment efficacy was constructed using LASSO Cox regression, incorporating five risk-associated genes: SEZ6, AKR1C1, LILRA5, NCKAP5, and SETD9 (Fig. 6f, g). Risk scores were calculated and validated by multivariate Cox regression, identifying NCKAP5 and SETD9 as significant independent predictors of survival (Fig. 6h). Kaplan-Meier and time-dependent ROC curves evaluated the predictive accuracy. Patients were divided into high-benefit and low-benefit groups based on median risk score, with Kaplan-Meier curves showing significantly better survival for the high-benefit group (Fig. 6i). The time-dependent ROC curves confirmed the accuracy of this model across multiple time points from 6 to 21 months (Fig. 6j).
Further validation using survival data from 418 advanced HCC patients in the TCGA-LIHC dataset showed no significant survival difference between high-benefit and low-benefit groups defined by this model (Fig. 6k). To facilitate clinical use, the authors developed an initial version of the predictive tool called Viropredict 1.0, available online at ViroPredict 1.0.
.webp)
Figure 6: Predictive Model of VG161 Efficacy in Advanced Hepatocellular Carcinoma (HCC)
VG161 is a promising third-line treatment for refractory HCC, offering both safety and therapeutic efficacy. It remodels the tumor immune microenvironment and performs comparably to second-line options. The newly developed predictive model helps identify patients most likely to benefit. Notably, antiviral medications did not affect VG161's replication or activity, supporting its use in HBV-infected populations. These findings underscore the need for further research and potential combination strategies to improve outcomes in advanced HCC.
Shen, Y., Bai, X., Zhang, Q. et al. Oncolytic virus VG161 in refractory hepatocellular carcinoma. Nature (2025).
https://doi.org/10.1038/s41586-025-08717-5
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong