
On March 28, 2025, Science published a peer-reviewed study by researchers from King Abdullah University of Science and Technology (KAUST), Australia’s CSIRO, and several collaborating institutions. The study reports a molecular mechanism by which wheat resists Puccinia graminis f. sp. tritici, the causal agent of stem rust. The findings identify a two-gene system involving a tandem kinase and an NLR protein that work in coordination to trigger pathogen-specific immune responses.
This research contributes to the broader understanding of plant immunity and may support future efforts to improve disease resistance in wheat through genetic approaches.
*Service Provider: Some yeast two-hybrid screening services were provided by Omics Empower. If you're looking for similar yeast library solutions—including library construction and screening—our team offers end-to-end support tailored to your research needs.
Stem rust remains one of the most damaging fungal diseases affecting global wheat production. In a previous study, the KAUST team cloned a resistance gene, Sr62TK, from Aegilops sharonensis, a wild wheat relative. Sr62TK encodes a tandem kinase that, when introduced into susceptible wheat lines, was shown to confer partial resistance.
However, further investigation revealed that Sr62TK alone was not sufficient to fully activate the immune response. Subsequent analysis identified a neighboring gene, now termed Sr62NLR, as a likely co-factor involved in the resistance mechanism.
Genetic mapping and functional assays confirmed that Sr62NLR is required for Sr62TK-mediated defense. In susceptible mutants, the Sr62TK sequence remained unchanged, but Sr62NLR was mutated and co-segregated with loss of resistance.
Complementation tests further demonstrated that both Sr62TK and Sr62NLR must be expressed together to elicit a specific immune response against the fungal effector AvrSr62.
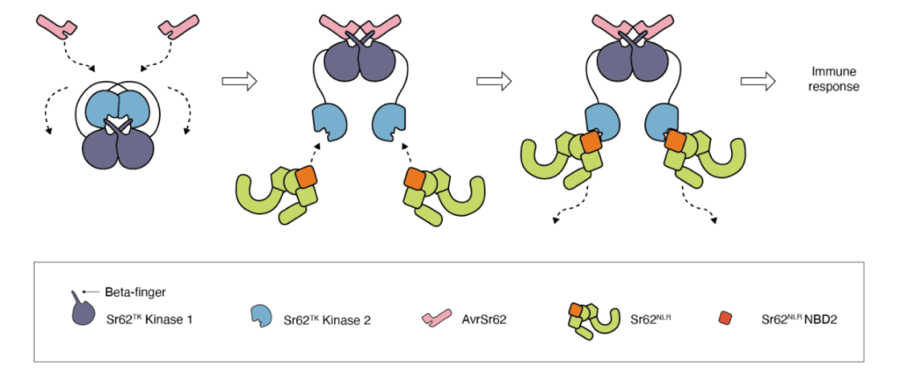
The researchers employed structural modeling and molecular biology techniques to describe a three-step activation model:
1. Effector Recognition: The Kinase1 domain of Sr62TK interacts directly with AvrSr62, as shown through yeast two-hybrid and co-immunoprecipitation assays.
2. Conformational Change and Signal Relay: Upon effector binding, Sr62TK undergoes structural changes that expose the Kinase2 domain, which then associates with Sr62NLR.
3. Immune Activation: This interaction initiates downstream immune responses. The conserved interface between Kinase2 and Sr62NLR suggests functional stability in the signaling process.
The study also found that Kinase1 is relatively variable, potentially allowing adaptation to pathogen evolution, while Kinase2 and Sr62NLR are more conserved, likely preserving signal reliability.
AlphaFold2 was used to predict the structure of the Sr62TK–Sr62NLR complex. The models suggested:
Kinase1 forms a homodimer through a β-strand interface.
Kinase2 mediates interaction with Sr62NLR, supporting conformational flexibility.
Site-directed mutagenesis targeting 21 residues helped validate key structural predictions. Notably, disrupting the Kinase1 dimer interface abolished protein interaction, while mutations in AvrSr62 altered its recognition, confirming the role of these domains in immune signaling.
Lu P. et al. (2025). A wheat tandem kinase and NLR pair confers resistance to multiple fungal pathogens. Science, 387, 1418–1424. DOI: 10.1126/science.adp5469
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong