Gastric adenocarcinoma (GA) is one of the most common and lethal cancers worldwide. Despite its prevalence, the disease remains poorly understood—mainly due to its unclear cellular origins and high degree of tumor heterogeneity. Because of their long lifespan, gastric stem cells are more likely to accumulate cancer-driving mutations that can lead to malignant transformation.
In the gastric antrum, LGR5⁺ basal stem cells are well-known markers of epithelial stem cells. Another important population, +4 stem cells marked by CCKBR (cholecystokinin B receptor), plays a key role in maintaining stem cell balance and responds to gastrin signaling. However, the role of CCKBR⁺ cancer cells—especially their heterogeneity and relevance to patient outcomes—has not been well characterized.
A study led by Prof. Hong Zheng at Anhui Medical University, published in Cell Death & Differentiation, reveals a critical role for CCKBR⁺ stem-like tumor cells in the progression of gastric adenocarcinoma.
Titled “CCKBR⁺ cancer cells contribute to the intratumor heterogeneity of gastric cancer and confer sensitivity to FOXO inhibition,” the paper uses single-cell RNA sequencing (scRNA-seq) to identify a distinct CCKBR⁺ subpopulation with stem cell–like features, a unique mutational profile, and strong links to poor patient prognosis.
Importantly, the study shows that FOXO signaling is essential for maintaining the stemness and survival of CCKBR⁺ gastric cancer cells, offering a promising direction for targeted therapies in gastric cancer.
*Omics Empower provided Single-cell transcriptome sequencing services.If you're looking for similar single-cell or spatial solutions support in your own research, our expert would be happy to help.
In this study, the authors used single-cell RNA sequencing (scRNA-seq) to identify a population of CCKBR⁺ stem-like tumor cells associated with poor differentiation and unfavorable prognosis in gastric adenocarcinoma (GA). These cells were shown to contribute significantly to the intratumoral heterogeneity observed in GA.
To validate their findings, the team performed targeted analyses using both their own scRNA-seq dataset (GSE183904) and publicly available data from The Cancer Genome Atlas (TCGA).
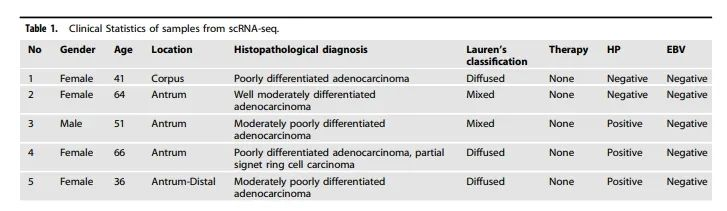
Table 1 summarizes the clinical characteristics of the samples analyzed via scRNA-seq.
The team conducted single-cell RNA sequencing (scRNA-seq) on tumor samples from five gastric adenocarcinoma (GA) patients, four gastric antrum cases, and one corpus cancer case. After quality control and outlier removal, they obtained a total of 24,931 cells, classified into eight major cell types. Focusing on epithelial cell clusters, they further identified 9 distinct tumor epithelial subpopulations via t-SNE clustering of 6,568 tumor epithelial cells. All clusters showed high malignancy scores.
One cluster in particular—Epi5—was characterized by high CCKBR expression, marking it as a +4 stem-like cell population from the gastric antrum. Epi5 cells lacked differentiation markers and instead expressed stemness-associated genes such as CD44, PROM1, and ALCAM, indicating strong cancer stem cell (CSC) potential.
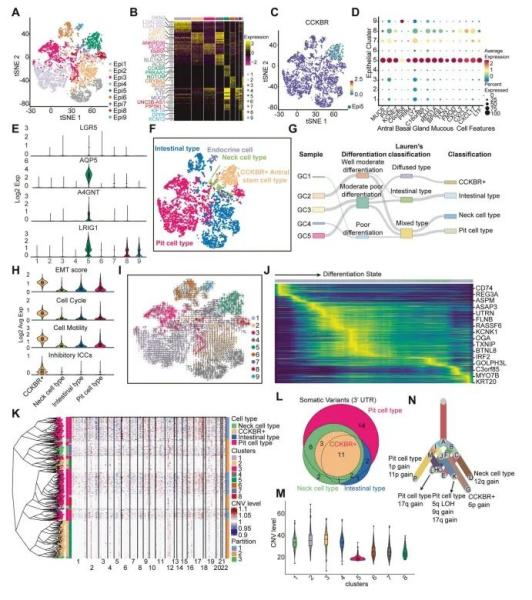
Figure 1. Single-cell RNA sequencing (scRNA-seq) identifies CCKBR⁺ stem-like tumor cells in gastric adenocarcinoma (GA)
To further investigate tumor heterogeneity, epithelial cells were classified into intestinal-type, foveolar-type, neck cell–type, CCKBR⁺ stem-like type, and minor endocrine subtypes. Among them, Epi5 cells were identified as the most primitive and poorly differentiated subtype, expressing high levels of aggressiveness-associated genes like CD74, REG3A, and ASPM.
To determine whether CCKBR⁺ cells originated from +4 stem cells or from dedifferentiation of other cancer types, the researchers analyzed copy number variations (CNVs) and evolutionary lineage in sample GC5. CCKBR⁺ cells showed a distinct CNV subclonal structure and the lowest CNV burden, supporting the view that they represent a separate lineage—not a dedifferentiated product of other cell types.
The authors performed immunohistochemical staining on 154 gastric tissue samples from the antrum and corpus to assess CCKBR expression across different levels of tumor differentiation. In normal gastric tissue, CCKBR is primarily expressed in the basal layer of antral glands, and its expression decreases progressively with cellular differentiation. In contrast, early-stage CCKBR⁺ tumor cells show strong expression in the lower regions of gastric glands, with expression expanding and spreading as the tumor progresses (Figure 2A).
CCKBR⁺ tumor cells were also identified in lymph node metastases, indicating metastatic potential of this population. Furthermore, CCKBR expression levels were significantly higher in CCKBR⁺ tumors compared to adjacent non-tumor tissues (Figure 2B).
When compared to the top five upregulated genes in GA, CCKBR demonstrated superior predictive sensitivity, suggesting its utility as a potential biomarker for gastric cancer prognosis. CCKBR⁺ tumors were mainly of antral origin and showed a strong association with poor differentiation (Figure 2C). These tumors also correlated with lower overall survival and worse post-treatment outcomes, likely due to a higher probability of residual microscopic tumor presence (Supplementary Figure 2C).
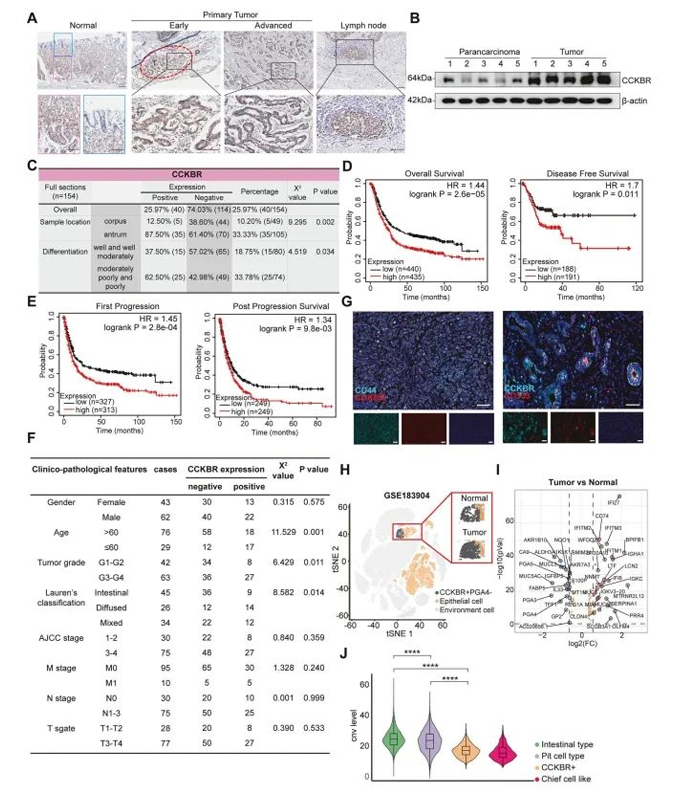
Figure 2. CCKBR expression is associated with poor differentiation and unfavorable prognosis in gastric adenocarcinoma
In addition to expression profiling, the authors conducted clinicopathological analyses comparing CCKBR-positive and CCKBR-negative gastric antral tumors. CCKBR⁺ tumors were significantly associated with:
younger patient age at diagnosis (χ² = 11.529, p = 0.001)
higher tumor grade (χ² = 6.429, p = 0.011)
and the Lauren diffuse subtype (χ² = 8.582, p = 0.014)
However, no significant association was observed between CCKBR status and TNM staging (Figure 2F).
The study also reported high co-expression of CCKBR with cancer stem cell markers CD44 and CD133 (Figure 2G), further supporting the stem-like identity of these cells as revealed by scRNA-seq.
To rule out sample bias, the authors validated their findings using an independent single-cell RNA-seq dataset (GSE183904), which included 40 gastric cancer and normal tissue samples. Analysis confirmed that CCKBR⁺ antral tumors exhibit lower levels of differentiation, higher malignant potential, and are associated with significantly poorer patient survival.
To investigate genomic alterations associated with CCKBR⁺ gastric tumors, the authors stratified tumor samples from The Cancer Genome Atlas (TCGA) based on CCKBR and Epi5 marker expression. These tumors displayed a distinct transcriptional profile, consistent with findings from the single-cell RNA-seq analysis.
Interestingly, CCKBR⁺ tumors exhibited significantly lower tumor mutation burden (TMB) and microsatellite instability (MSI) scores compared to other subtypes. The average mutation count per tumor was markedly reduced (193 vs. 602). According to TCGA classification:
The incidence of MSI subtype was reduced in CCKBR⁺ tumors (10.47% vs. 30.94%)
EBV-associated tumors were absent (0% vs. 10.07%)
In contrast, genomically stable (GS) tumors were more frequent (16.28% vs. 8.63%)
Most notably, the proportion of chromosomal instability (CIN) subtype was substantially higher (72.09% vs. 48.92%)
l(Figure 3C)
A similar trend was observed when tumors were re-classified using canonical stem cell markers, including LGR5, AQP5, A4GNT, and LRIG1, reaffirming that stem-like tumor cells are more likely to give rise to GS and CIN subtypes rather than MSI or EBV types (Figure 3D).
Further genomic profiling revealed that CCKBR⁺ tumors are frequently driven by mutations in TP53, GLI3, APC, and SMAD4—mutational patterns that align with the development of CIN and GS subtypes (Figure 3H, 3J).
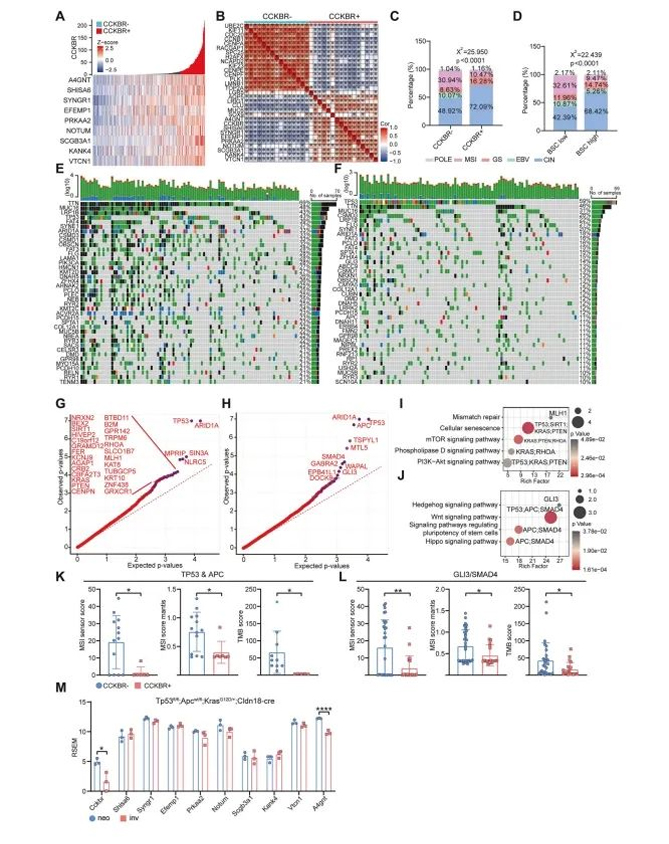
Figure 3. CCKBR⁺ tumors are enriched in chromosomal instability (CIN) and genomically stable (GS) subtypes
To determine whether TP53, APC, GLI3, and SMAD4 mutations directly contribute to the formation of CCKBR⁺ tumors, the authors analyzed genomic data from CCKBR-positive and CCKBR-negative patient samples carrying these mutations. They compared copy number variation (CNV) profiles across groups.
Despite the presence of similar driver mutations, CCKBR⁺ tumors consistently exhibited lower TMB and MSI scores than their CCKBR⁻ counterparts (Figure 3K, 3L), suggesting that these mutations alone are insufficient to account for the CCKBR⁺ tumor phenotype.
To further validate this, the authors examined a mouse model of gastric adenocarcinoma induced by TP53, APC, and KRAS mutations. Notably, CCKBR and Epi5-associated markers were not upregulated in these models (Figure 3M).
Taken together, the results suggest that while classic driver mutations are necessary, they are not sufficient to induce the stem-like CCKBR⁺ tumor phenotype. Instead, malignant transformation must occur specifically within gastric stem cells, as the same genetic alterations in non-stem cell contexts fail to give rise to dedifferentiated, CCKBR⁺ tumors.
To explore the biological characteristics of CCKBR⁺ tumor cells, the authors performed functional enrichment analysis on the Epi5 cluster—the subpopulation identified as stem-like.
Epi5 cells were primarily associated with pathways involved in transcriptional initiation, autophagy, cell adhesion, hypoxic response, and immune regulation (Figure 4A and Supplementary Figures 4A, 4B). These signatures suggest that CCKBR⁺ cells exist in a hypoxic tumor microenvironment and rely on autophagy and adhesion mechanisms for survival and proliferation.
Notably, Epi5 cells did not exhibit strong proliferative signaling, but rather were regulated by transcription factors linked to inflammation (e.g., NFKB1, RELA, STAT3, ZFP36) and stemness (e.g., JUN, FOXO3, FOXO4, CTNNB1) (Figure 4B).
Pathway enrichment analysis further revealed activation of several signaling cascades, including:
NF-κB, TNF, and IL-17 pathways (inflammatory)
FOXO, Hippo, HIF-1, and TGF-β pathways (stem cell–associated)
Importantly, the FOXO signaling pathway was markedly enriched in Epi5 cells (Figure 4C), highlighting its potential role in sustaining stem-like traits in CCKBR⁺ gastric cancer cells.
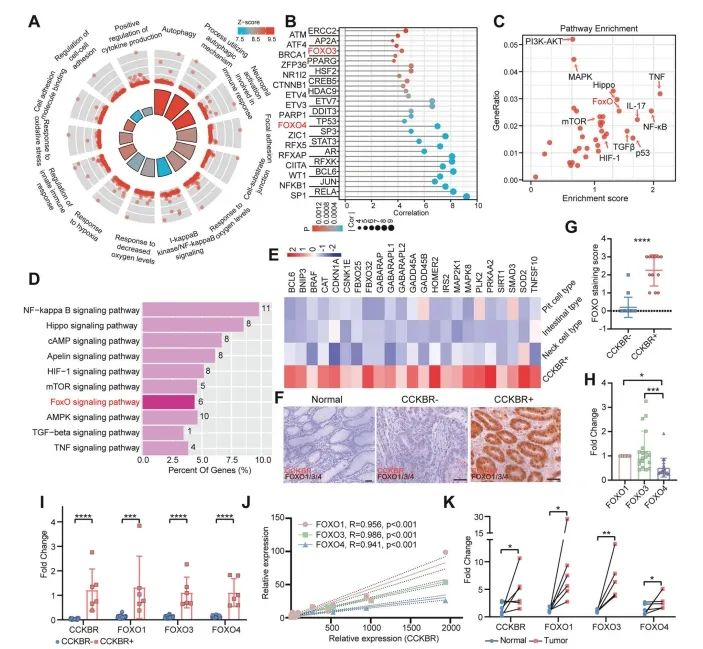
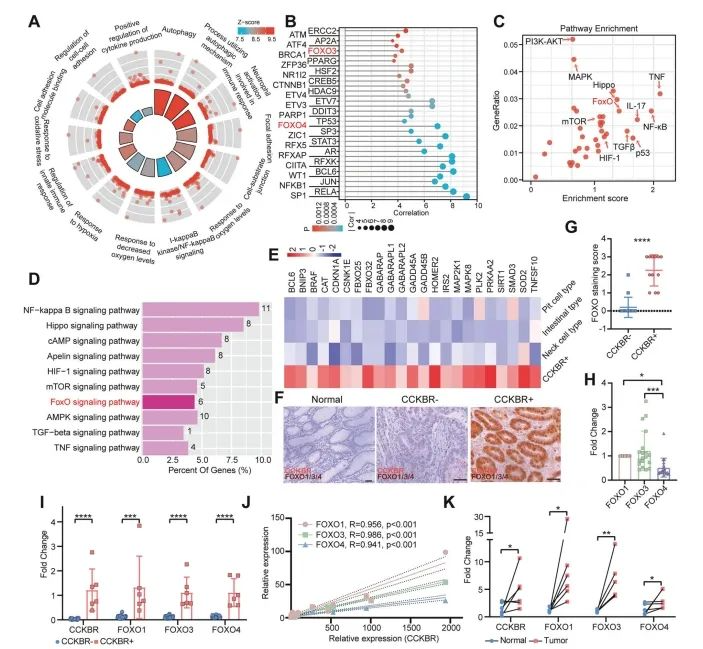
Figure 4. Enhanced FOXO pathway activation in CCKBR⁺ tumors
To validate FOXO pathway enrichment in an independent dataset, the authors analyzed GSE183094, which includes a broader set of gastric cancer and normal samples. They specifically identified a subpopulation of CCKBR⁺PGA4⁻ tumor cells with enriched FOXO pathway signatures (Figure 4D).
Across both Epi5 cells and CCKBR⁺ tumor cells from GSE183094, genes involved in FOXO signaling were consistently upregulated, supporting the finding that FOXO activity is a hallmark of the CCKBR⁺ stem-like tumor phenotype (Figure 4E).
To assess the functional relevance of FOXO signaling in CCKBR⁺ tumor cells, the authors compared CCKBR-positive and -negative cell populations in tumorsphere formation assays. Results showed that CCKBR⁺ cells had a markedly greater capacity for tumorsphere generation, indicating higher stemness potential (Figure 5B, 5C).
To further evaluate their cancer stem cell (CSC) content, a limiting dilution transplantation assay was performed using HGC27 gastric cancer cells sorted for CCKBR expression and injected into immunodeficient mice. CCKBR⁺ cells formed significantly larger tumors compared to their CCKBR⁻ counterparts (Figure 5D–G and Supplementary Figure 5B).
In vitro, tumorsphere culture led to a substantial upregulation of CCKBR and FOXO1/3/4 proteins across six different gastric cancer cell lines, compared to standard adherent culture (Figure 5H, 5I). Additionally, expression of FOXO1, FOXO3, and FOXO4 was consistently elevated in tumorspheres (Figure 5J, 5K), further supporting their role in maintaining CSC-like properties in CCKBR⁺ cells.
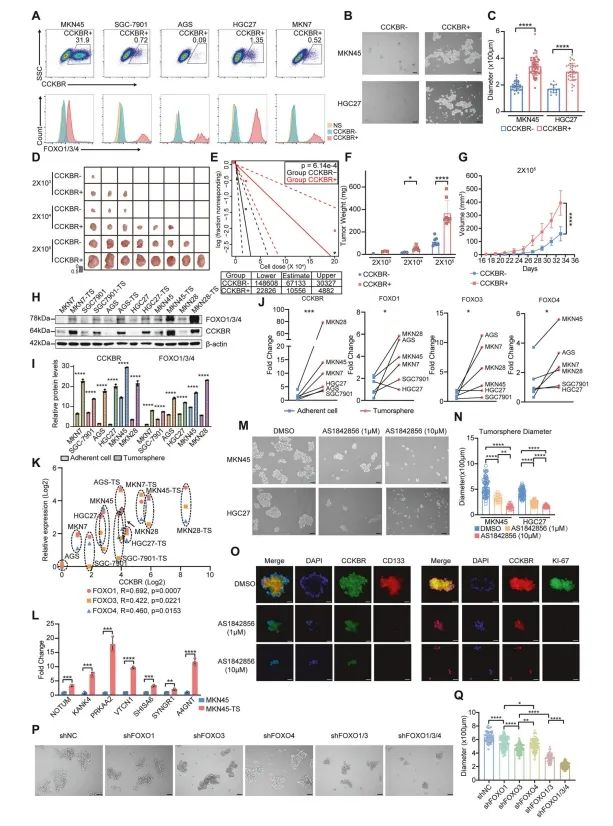
Figure 5. FOXO transcription factors maintain the stem-like state of CCKBR⁺ gastric cancer cells
Further supporting the stem-like nature of CCKBR⁺ tumor cells, the authors found that Epi5-associated markers were significantly upregulated in tumorspheres derived from these cells (Figure 5L).
To evaluate the functional role of FOXO signaling, tumorsphere cultures were treated with varying concentrations of AS1842856, a selective FOXO inhibitor. Treatment with 1 μM AS1842856 significantly suppressed tumorsphere formation. In addition, RT-qPCR analysis confirmed the downregulation of Wnt pathway genes, gastric stem cell markers, and CSC markers following FOXO inhibition (Supplementary Figure 5E).
To dissect the individual contributions of FOXO transcription factors, the authors generated FOXO1, FOXO3, and FOXO4 knockout lines in MKN45 gastric cancer cells. Single-gene knockouts only modestly reduced tumorsphere formation. However, dual knockout of FOXO1 and FOXO3 resulted in a marked decrease in sphere formation, and triple knockout of FOXO1/3/4 produced an even greater inhibitory effect (Figure 5P, 5Q).
These results suggest that compensatory mechanisms among FOXO isoforms exist, and that simultaneous inhibition of multiple FOXO family members may be necessary to effectively suppress CSC activity in CCKBR⁺ tumors.
To directly compare the drug response between CCKBR⁺ and CCKBR⁻ tumor cells, the authors established gastric cancer organoids from cells with or without CCKBR expression (Figure 6A). Consistent with earlier results, CCKBR⁺ organoids showed elevated expression of CCKBR and stem cell markers.
Organoids have been shown to retain the drug response profile of primary tumors. In this model, treatment with AS1842856 significantly suppressed the growth of CCKBR⁺ organoids. Specifically, 1 μM AS1842856 reduced cell viability, and higher doses exerted cytotoxic effects on CCKBR⁺ gastric adenocarcinoma (GA) organoids. In contrast, CCKBR⁻ organoids were largely unaffected (Figure 6B, 6C).
The authors also tested carbenoxolone, a novel FOXO pathway inhibitor, which likewise showed robust suppression of CCKBR⁺ GA organoid growth (Figure 6D).
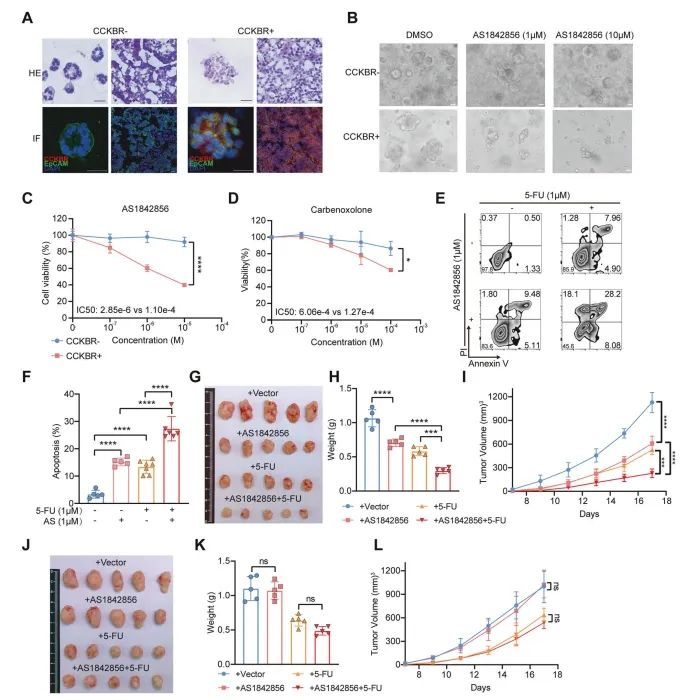
Figure 6. Targeting FOXO suppresses the growth of CCKBR⁺ tumor cells
In addition to organoid inhibition, the authors explored whether combining FOXO inhibition with chemotherapy could further enhance anti-tumor effects. Treatment of CCKBR⁺ tumor cells with both AS1842856 and 5-fluorouracil (5-FU) led to a significant increase in apoptosis, compared to either agent alone (Figure 6E, 6F).
To assess this combination strategy in vivo, the authors xenografted CCKBR⁺ MKN45 cells into immunodeficient mice and treated them with AS1842856, 5-FU, or both. AS1842856 alone reduced tumor growth, while the combination therapy produced a more pronounced inhibitory effect on tumor size (Figure 6G–I).
In contrast, the same treatments had minimal effect on AGS-derived tumors, which are CCKBR-negative, indicating that the therapeutic benefit is specific to CCKBR⁺ stem-like tumor cells (Figure 6J–L).
Taken together with in vitro findings, these results suggest that FOXO inhibition selectively suppresses the growth of CCKBR⁺ gastric cancer cells, especially in combination with chemotherapeutic agents like 5-FU.
To investigate the downstream mechanisms by which FOXO signaling sustains CCKBR⁺ tumor cells, the authors conducted high-throughput CUT&Tag sequencing on CCKBR⁺ and CCKBR⁻ tumor cells. As expected, binding motifs for FOXO1, FOXO3, and FOXO4 were enriched, suggesting that these transcription factors share common regulatory elements (Figure 7A).
Differential peak analysis of promoter regions revealed that FOXO targets promoted tumor cell survival and enhanced hypoxia responses, consistent with earlier observations of FOXO pathway activation in a hypoxic microenvironment (Figure 7B).
Among the downstream genes, the ST3GAL sialyltransferase family (α2,3-sialylation) was markedly upregulated in CCKBR⁺ cells, while ST6GALNAC family members (α2,6-sialylation) were not. Aberrant sialylation is a known driver of tumor progression, metastasis, and immune evasion.
RT-qPCR confirmed that ST3GAL genes were significantly elevated in CCKBR⁺ GA organoids, and importantly, their expression was suppressed by AS1842856 treatment (Figure 7I). These findings were further validated by MAL II lectin staining, which showed reduced α2,3-sialic acid after FOXO inhibition (Figure 7J).
Finally, pharmacological inhibition of α2,3-sialylation using P3FAX-Neu5Ac led to significant suppression of tumor growth in CCKBR⁺ cells, while having minimal impact on CCKBR⁻ cells (Figure 7K).
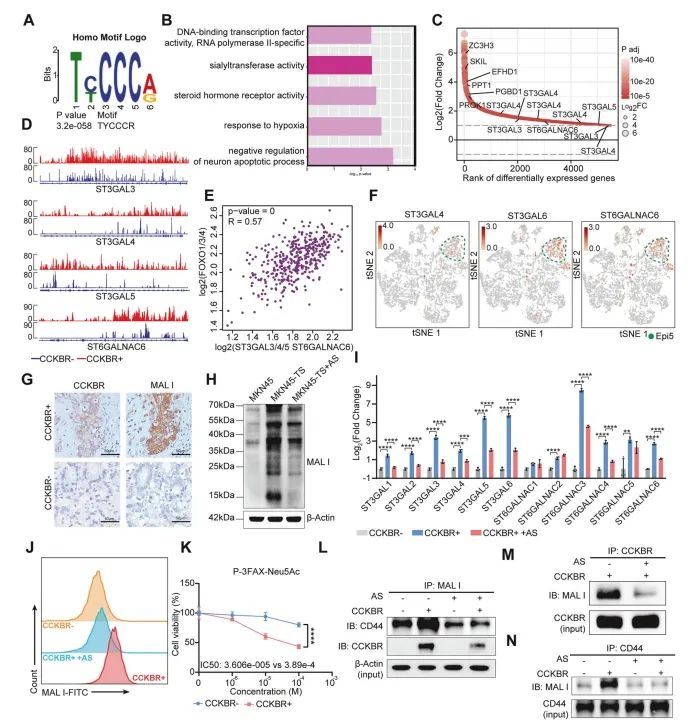
Figure 7. FOXOs transcriptionally regulate α2,3-sialylation and promote the growth of CCKBR⁺ tumors
In summary, the study demonstrates that FOXO transcription factors promote the expression of key sialyltransferases—including ST3GAL3, ST3GAL4, ST3GAL5, and to a lesser extent, ST6GALNAC6—in CCKBR⁺ gastric cancer cells. This upregulation leads to elevated levels of α2,3-linked sialoglycans on critical surface molecules such as CD44 and CCKBR, enhancing tumor growth and potentially contributing to immune evasion and metastasis.
The heterogeneous phenotypes of gastric tumors contribute to variable drug responses, ultimately limiting the effectiveness of standardized treatment approaches. In healthy gastric tissue, long-lived stem cells play a central role in epithelial maintenance. Both basal stem cells and +4 stem cells have been implicated as potential cells of origin for antral gastric cancer through cell-type–specific oncogenic mutations.
In this study, the authors used single-cell transcriptomic profiling to identify a CCKBR-expressing +4 stem cell–like subpopulation in the distal stomach. These cells were characterized by poor differentiation, high malignancy, and association with reduced patient survival.
Compared to foveolar- or intestinal-type tumor cells, CCKBR⁺ cancer cells showed strong activation of inflammatory, autophagic, and stemness-associated signaling pathways. The study also revealed that FOXO signaling is essential for maintaining the malignant and stem-like characteristics of this tumor subtype.
Moreover, the authors identified a FOXO–sialyltransferase axis that promotes tumor progression by enhancing α2,3-linked sialylation on surface proteins like CD44 and CCKBR, facilitating CCKBR-driven cancer stem cell growth and survival (Figure 8).
This work provides novel insight into the molecular basis of tumor heterogeneity in gastric adenocarcinoma and highlights CCKBR⁺ stem-like tumor cells as a promising therapeutic target. Targeting FOXO signaling and its downstream glycosylation programs may open new avenues for precision treatment strategies in gastric cancer.
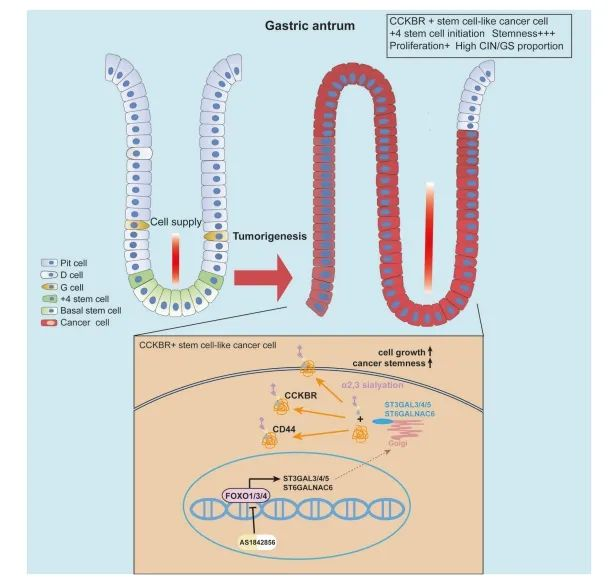
Figure 8. CCKBR⁺ cancer cells in gastric adenocarcinoma exhibit stem-like properties
Tan, Z., Pan, K., Sun, M. et al. CCKBR+ cancer cells contribute to the intratumor heterogeneity of gastric cancer and confer sensitivity to FOXO inhibition. Cell Death Differ 31, 1302–1317 (2024). https://doi.org/10.1038/s41418-024-01360-z
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong
Germany: Arnold-Graffi-Haus / D85 Robert-Rössle-Straße 10 13125 Berlin
United States: (CA) 2 Goddard, Irvine, CA 92618
United States: (IL) 8255 Lemont Rd, #1, Darien, IL 60561
Hong Kong: Room 618, Building 6, Hong Kong Science Park, Pak Shek Kok, Hong Kong